Introduction
Implementing a clinical data management
system (CDMS) involves a multitude of decisions at all
levels. Data managers face the intimidating task of
getting consensus around literally thousands of issues.
Data standards must be established, a thesaurus put
in place, input screens created - and all the parts
need to work together. It's a mountain of detail, and
with each step upward the clock is ticking.
Clinical directors and vice presidents
face an equally mountainous task: they need to ensure
that everything possible is done to maximize benefits
from the new CDMS. Decisions abound, how will the CDMS
relate to legacy systems? How will redundant data in
multiple systems be reconciled? What about concurrent
or future initiatives such as an Electronic Data Capture
(EDC) system? And above all, how does this system get
deployed on time, and on budget?
With Quintegra’s CDMS, you can
energize your clinical data management process. The
CDMS is at the heart of your clinical research machine,
the engine that drives projects to the earliest possible
data lock.
Offering
The Healthcare Practice at Quintegra
provides smart and innovative solutions to several leading
healthcare organizations around the world. The Healthcare
Practice provides a revolutionary CDMS for effective
and efficient management of clinical data management
process.
A CDMS will bring the efficiency of
your clinical trials process to an entirely new level.
That's why you've decided to go for a CDMS. Now you
face several challenges: making the system work, getting
it done on time, and fitting it into your company's
broader technology strategy. Some of these responsibilities
may require resources you just don't have available.
Quintegra offers a comprehensive CDMS
for making sure software and business processes work
together. We have the broad business perspective to
support your company in making the big decisions that
bring you the greatest benefit, and the operational
and technology skills to make the myriad pieces fall
smoothly into place. Most important, we have in-depth,
hands-on knowledge of clinical data management processes.
Quintegra’s CDMS is a centralized
clinical data management and analysis system that will
assist clinical investigators in managing protocols
and patient & research data as well as in integrating
disparate data sources for analysis.
The components of Quintegra’s CDMS are:
 |
A
Protocol Tracking Management System (PTMS) that
supports protocol submission, approval, and monitoring
of protocol review process. |
 |
A Clinical
Study Informatics System (CSIS) that provides
patient data management via protocol setup for
patient recruitment, screening, enrollment, dynamic
form creation, data collection, monitoring, and
reporting. |
 |
A data integration
module that provides data warehousing services
to collect data from a variety of data sources
such as external scientific databases; its scope
also encompasses analytics tools that will facilitate
data mining and statistical analysis of data from
CDMS as well as the externalsources to support
biomedical discovery and translational research.
|
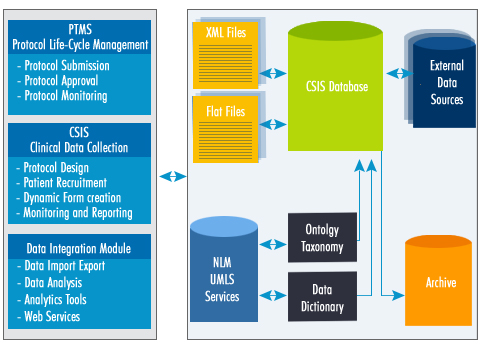
Quintegra’s
Clinical Data Management System (CDMS) Overview Schematic
The CDMS is a Web-based and platform-independent system
with a back-end relational database management system.
It employs a multi-tiered architecture in which each
tier can be executed on separate hardware platforms.
Quintegra has adopted several industry-leading frameworks
to accelerate development time and improve maintainability.
Core frameworks include Microsoft .NET, IBM WebSphere
and ObJect-relational Bridge (OJB).
Once you have invested in Quintegra’s
CDMS, you want to see payoff as quickly as possible.
Quintegra has the project management capabilities to
make it happen. We put teams in place, and create goals,
objectives, and critical path-based timelines. You are
kept informed and aware of progress and issues, while
we work to be certain issues are resolved rapidly. Your
company will be able to quickly move studies into a
production environment. We use our understanding of
business requirements to adapt your existing clinical
data management process to work with system changes,
or to customize the application to fit unique business
process requirements.
The CDMS is only the starting point for handling the
flood of clinical data for the drug development pipeline.
CDMS implementation often leads to broader strategic
questions about the clinical data management infrastructure.
As a value-add, Quintegra has the vision and expertise
in technology and the clinical drug research environment
to help answer these critical questions. We look to
exploit the opportunities offered by the Internet to
break down data silos, using technology architecture
to create a collaborative systems environment that supports
new approaches to every aspect of the clinical research
and marketing processes.
Quintegra’s CDMS
allows for:
 Faster trial design and planning processes
Faster trial design and planning processes
 More efficient collection and management of both patient
and trial administration data
More efficient collection and management of both patient
and trial administration data
 Improved FDA compliance
Improved FDA compliance
 Secure, centralized access to sites, trials, and programs
Secure, centralized access to sites, trials, and programs
 High accuracy data entry
High accuracy data entry
 Comprehensive data validation
Comprehensive data validation
 Full electronic Audit Trail
Full electronic Audit Trail
Our understanding of data systems throughout the pharmaceutical
enterprise lets us build information bridges to create
new value for you from existing data resources. Quintegra’s
advanced technical expertise and the CDMS solution make
us the choice to assist your company complete a robust,
documented CDMS implementation and integration.

|
